The cosmetics industry in the United States is a multi-billion-dollar market, producing everything from skincare and haircare to fragrances and personal care products. While cosmetics do not require FDA pre-market approval, they are still subject to strict regulatory oversight to ensure product safety, accurate labeling, and proper manufacturing practices.
FDA Compliance & Cosmetics
Unlike drugs or medical devices, cosmetics do not require FDA approval before being sold. However, the FDA has enforcement authority to regulate cosmetic products and take action against misbranded or adulterated cosmetics that pose health risks. Compliance with FDA regulations ensures that products:
- Are properly labeled with accurate ingredient lists and claims that are not misleading.
- Do not contain prohibited or restricted ingredients (such as mercury or certain color additives).
- Are manufactured under sanitary conditions to prevent contamination.
- Are safe for consumer use, with proper testing and adverse event monitoring in place.
For years, FDA regulations on cosmetics remained relatively unchanged—until the Modernization of Cosmetics Regulation Act (MoCRA) was signed into law in December 2022. MoCRA represents the most significant expansion of FDA oversight in decades, placing new requirements on manufacturers, packagers, and distributors of cosmetic products.
MoCRA Compliance: What You Need to Know
MoCRA is here, and it’s reshaping the U.S. cosmetics industry. This law expands the FDA’s authority over cosmetics and personal care products, ensuring greater oversight of manufacturing, product safety, and labeling.
Key MoCRA Requirements:
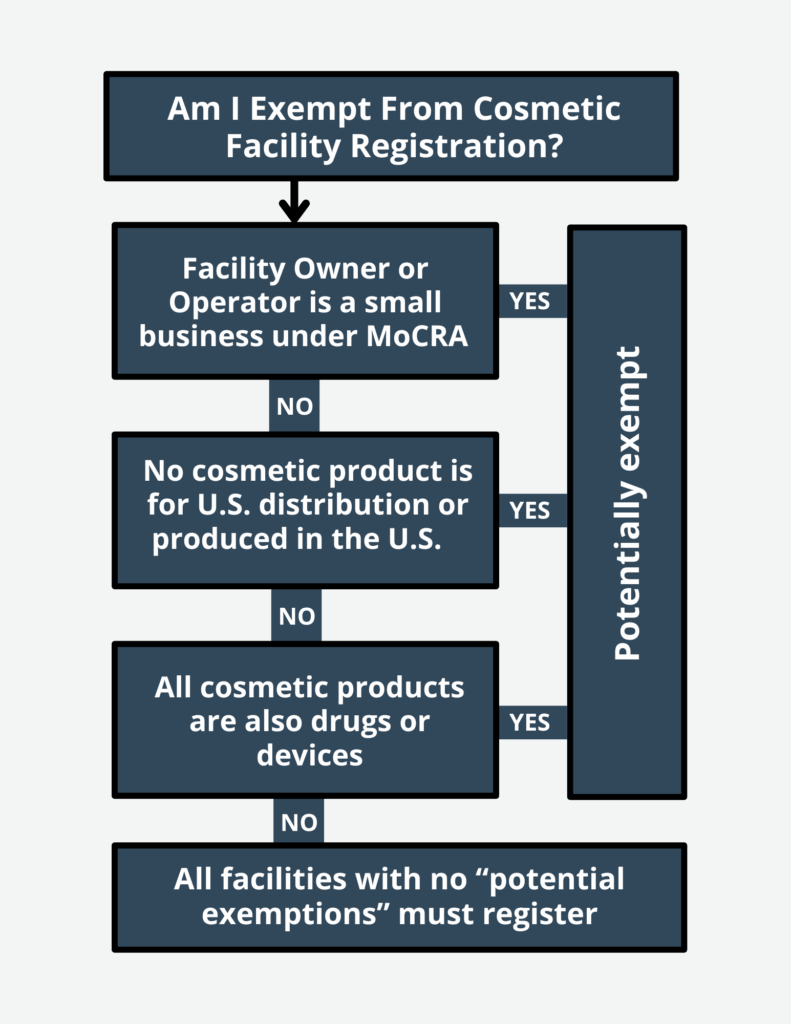
✔ Facility Registration – Cosmetic manufacturers and processors must register their facilities with the FDA, update changes within 60 days, and renew registration every two years.
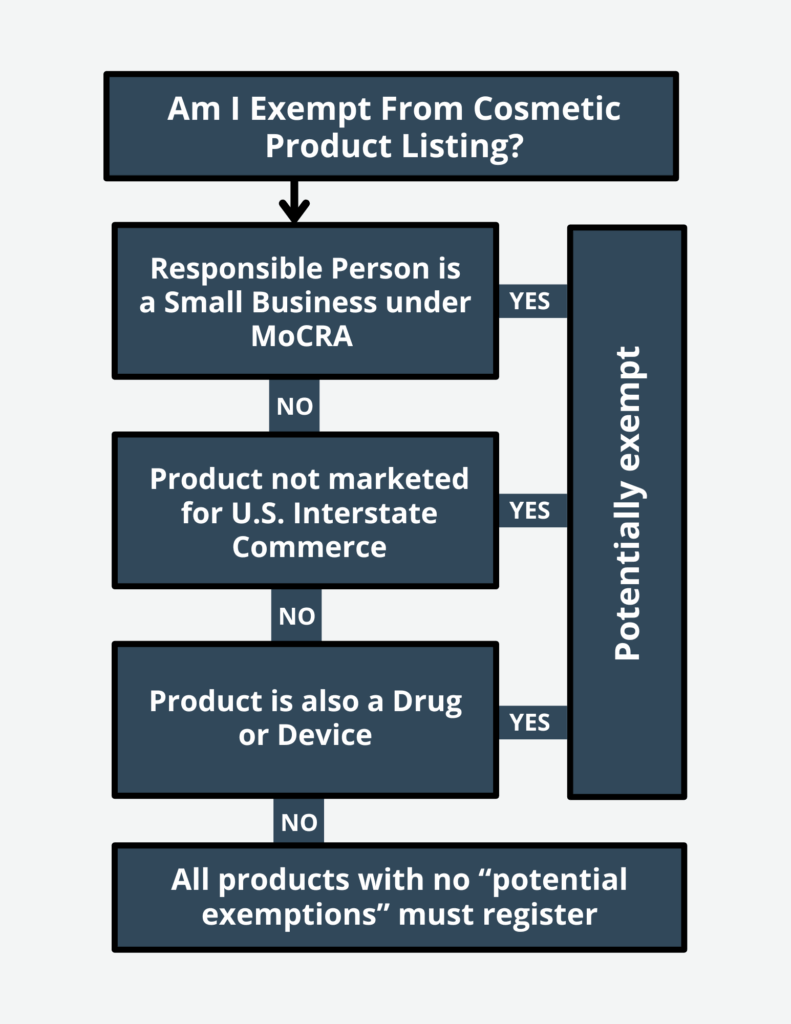
✔ Product Listing – Each marketed cosmetic product must be listed with the FDA, including an ingredient list, and updated annually.
✔ Adverse Event Reporting – Manufacturers must report serious adverse events (e.g., hospitalizations, disfigurement) to the FDA within 15 business days and maintain records for six years (three years for small businesses).✔ Good Manufacturing Practices (GMP) – By December 29, 2025, the FDA will introduce mandatory GMP regulations for cosmetics, aligning them with existing standards for drugs and medical devices.
✔ Good Manufacturing Practices (GMP) – By December 29, 2025, the FDA will introduce mandatory GMP regulations for cosmetics, aligning them with existing standards for drugs and medical devices.
Who Needs to Comply with MoCRA & Other FDA Regulations?
If you operate in the cosmetics industry, compliance with MoCRA and other FDA regulations is mandatory for certain business categories:
✔ Manufacturers – Companies producing cosmetics for U.S. distribution.
✔ Packagers – Businesses responsible for packaging cosmetics (if also the manufacturer).
✔ Distributors – Firms marketing cosmetics under their own brand, even if another company manufactures the product.
✔ Contract Manufacturers – Third-party manufacturers producing private-label cosmetics must collaborate with brand owners for compliance.
How Our MoCRA Compliance Consultants Can Help Navigate FDA & MoCRA Requirements
Navigating FDA compliance and MoCRA requirements can be complex and time-consuming. Our team of regulatory experts provides comprehensive services to help your business meet federal requirements and maintain compliance.
✅ Regulatory Compliance Services – Stay up to date with FDA regulations, including facility registration, product listing, and GMP requirements.
✅ Labeling & Packaging Reviews – Ensure your cosmetic labels meet FDA guidelines, avoiding compliance risks.
✅ MoCRA Compliance Support – From individual services to customized compliance packages, we help streamline your MoCRA submissions.
Pricing for MoCRA Compliance Services
| 1-50 Labels | $4,000 |
| 51-100 Labels | $7,500 |
| 101-500 Labels | $12,500 |
What Our MoCRA Compliance Consultants Need From You
📌 Product Labels – Upload in PDF format for review.
📌 Basic Business Information – Complete the MoCRA compliance form, including legal name, address, phone number, and email.
MoCRA Compliance Terms to Know
FDA (Food and Drug Administration) – The federal agency responsible for regulating food, drugs, and cosmetics in the U.S.
Responsible Person – The entity whose name appears on a cosmetic product label and is responsible for ensuring MoCRA compliance.
Good Manufacturing Practices (GMP) – FDA-enforced quality control standards ensuring safe and consistent cosmetic products.
Adverse Event Reporting – The process of reporting harmful reactions or safety concerns related to a cosmetic product to the FDA.
Serious Adverse Event (SAE) – A reaction to a cosmetic product that results in hospitalization, disfigurement, disability, or death, requiring FDA notification within 15 business days.
Facility Registration – Mandatory registration of all cosmetic manufacturing facilities with the FDA, ensuring regulatory oversight.Product Listing – The FDA’s requirement that all marketed cosmetics be registered with an ingredient list and updated annually.
Stay Compliant & Confident in the Cosmetics Industry
The cosmetics industry is undergoing significant changes, and compliance with FDA regulations is crucial for long-term success. Whether you need help with MoCRA compliance, product labeling, or regulatory approvals, our experts are ready to guide you.
